The decision after resisting it for months due to lack of peer reviewed data, is expected to pave the way for the local government to authorise its use in the city.
Hong Kong government had last month decided to exempt Sinovac from having to publish third phase clinical trial data in medical journals for peer review, due to the “urgency” for vaccination.
The Pfizer/BioNTech vaccine the only formally approved Covid-19 vaccine in Hong Kong for now, was required to have published results in a medical journal before being examined by the advisory panel.
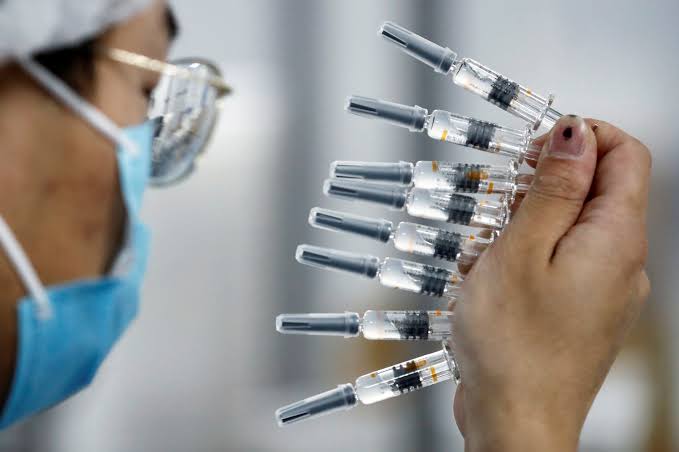
The panel’s convenor, Professor Wallace Lau, said the panel unanimously agreed that the vaccine’s efficacy outweighs its possible risks, after receiving data from the mainland drug maker suggesting that its product’s efficacy rate exceeds 60 percent when two doses are administered 28 days apart.
A previously-reported efficacy rate of barely over 50 percent from late-stage clinical trials in Brazil had raised concerns that the vaccine isn’t as effective as it should be for emergency use.
The World Health Organisation (WHO) is yet to approve the Sinovac vaccine. The panel also denied any pressure from the government into recommending the vaccine.
Sinovac was supposed to have already delivered a million doses of coronavirus vaccines to Hong Kong by the end of January, but authorities were unable to approve the jabs because of a lack of the necessary data.




















 WhatsApp us
WhatsApp us