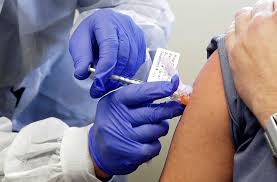
The first two volunteers in the human trial for a vaccine against Covid-19 were innoculated at the University of Oxford on Thursday evening, with experts expressing cautious optimism and much of the world anticipating its success.
Sarah Gilbert, professor of vaccinology at the university, is hopeful of developing the vaccine by September, with initial results expected by mid-May. The Oxford project is one of nearly 80 such initiatives across the globe to develop a vaccine for Covid-19.
One of the two innoculated on Thursday was named as Elisa Granato, a microbiologist.
As the trial was rolled out on Friday on more volunteers, the university sounded a note of caution: “A high proportion of vaccines are found not to be promising even before clinical trials. Moreover, a significant proportion of vaccines that are tested in clinical trials don’t work”.
“If we are unable to show that the vaccine is protective against the virus, we would review progress, examine alternative approaches, such as using different numbers of doses, and would potentially stop the programme”, it said.
The two volunteers are among over 1,100 between the ages of 18 and 55 recruited for the trial in Oxford, Bristol, Southampton and London. Half of them receive the vaccine and the other half (the control group) receive a widely available meningitis vaccine.
The vaccine produced in the university is based on an adenovirus vaccine vector and the SARS-CoV-2 spike protein, intended to test against Covid-19 in healthy volunteers. It aims to assess whether healthy people can be protected from COVID-19 with the vaccine called ChAdOx1 nCoV-19.
ChAdOx1 nCoV-19 is made from a virus (ChAdOx1), which is a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees, that has been genetically changed so that it is impossible for it to grow in humans.
The volunteers are given an E-diary to record symptoms for a week after receiving the vaccine and attend a series of follow-up visits, when a blood sample will be taken and the E-diary reviewed. The blood samples will be used to assess the immune response to the vaccine.
“If participants develop COVID-19 symptoms during the study, they can contact a member of the clinical team, and we will assess them to check whether they have become infected with the virus. If a participant was very unwell, we would call our colleagues in the hospital and ask them to review the volunteer if appropriate”, the university added.

























 WhatsApp us
WhatsApp us