Anti-viral drug Favipiravir approved for treatment of mild to moderate COVID-19 patients will be commercialised full scale in a week, says drug maker Glenmark Pharmaceuticals.
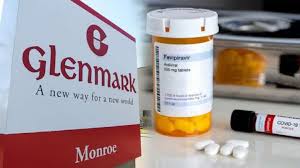
Glenmark’s Fabiflu is the first oral Favipiravir-approved medication for the treatment of COVID-19 and has been launched in few centers. The Drug Controller General of India approved the anti-viral for ‘restricted emergency use’ for treatment of mild to moderate cases, which accounts for a majority of COVID-19 infections.
Fabiflu, available as a 200 mg tablet, has been priced at Rs 103 per tablet. A strip of 34 tablets will cost Rs 3,500 to the patients. The recommended dosage duration has been fixed for 14 days. On day 1, a dose of 1,800 mg twice a day is recommended, followed by 800 mg twice a day from day 2 up to 14th day. The full 14 day treatment cycle will cost Rs 14,000 to patients.
“The drug will be available both in hospitals and in retail chemist stores by next week and will be available only on a doctor’s prescription, who will be first taking patients’ consent before starting the the drug,” said Sujesh Vasudevan, president – India Formulations, Glenmark Pharmaceuticals.
As a standard requirement of restricted emergency use, a written consent will be taken from patients. A copy of the form is placed in the pack of Fabiflu, says the company. Favipiravir will be manufactured in Glenmark’s facility in Baddi. The company, however, refrained from sharing any details about the manufacturing capacity or volumes it anticipates to supply.
The drug should not be prescribed to patients with severe renal, hepatic impairment, pregnant and lactating women. The drug is also contraindicated in patients with history of hypersensitivity to Favipiravir and those with history of having Gout or uric acid imbalances.
DCGI’s approval was based on Glenmark’s phase 3 clinical trials of a randomized, multi-centric study at 11 sites in India to test efficacy and safety of Favipiravir.
Monika Tandon, vice president and head, clinical development at Glenmark, in a web press conference said of the 150 COVID-19 patients enrolled, 90 were mild and 60 were moderate patients said their conditions had not deteriorated to severe or critical stages of the disease.
Starting the drug early is key, Tandon added, “High rate of viral replication can be controlled with early use of antiviral drugs and it is seen to limit immune response mediated damage as immune response subsides quickly.”
The details of the trial results are yet to be published, but the company said most patients gradually recovered in a week. Others took almost two weeks to recover.
Nearly 18 global clinical trials in over 3,000 patient subjects have been conducted globally. Tandon said while Glenmark’s trial was on 150 patients, the data was corroborated with other trials in USA, Canada, Italy, China, France and UK.
Favipiravir is also approved for compassionate use in Japan and nearly 2,050 patients have already been administered Favipiravir. “Japan’s clinical use showed high recovery rates at both 7 days (74 percent) and 14 days (88 percent) of therapy in both mild and moderate patients.”
Another study in 390 patients in Russia showed 68 percent of those on Favipiravir reached fever resolution on day 3 versus day 6 for those on standard therapy. Favipiravir is also approved by Italy and China for experimental use/compassionate use in COVID-19 and few countries in the Middle East.
Glenmark is now embarking on a second trial for combination of two anti-viral drugs Favipiravir (Approved drug for novel flu pandemics) with Umifenovir (Approved drug for Influenza). The safety of this combination is well established said the company.
Amidst reports that multiple other drug manufacturers are awaiting marketing approvals for Favipiravir, Glenmark’s Vasudevan said he does not anticipate competition in India anytime soon. While working to expand supplies in India, Glenmark is also exploring exports of Favipiravir to markets in the Middle East, where some countries have approved the drug for treating COVID-19 patients.Reports say Hyderabad-based drug maker Optimus Pharma has started shipping the antiviral tablet Favipiravir to the UAE, where the drug is approved.

























 WhatsApp us
WhatsApp us