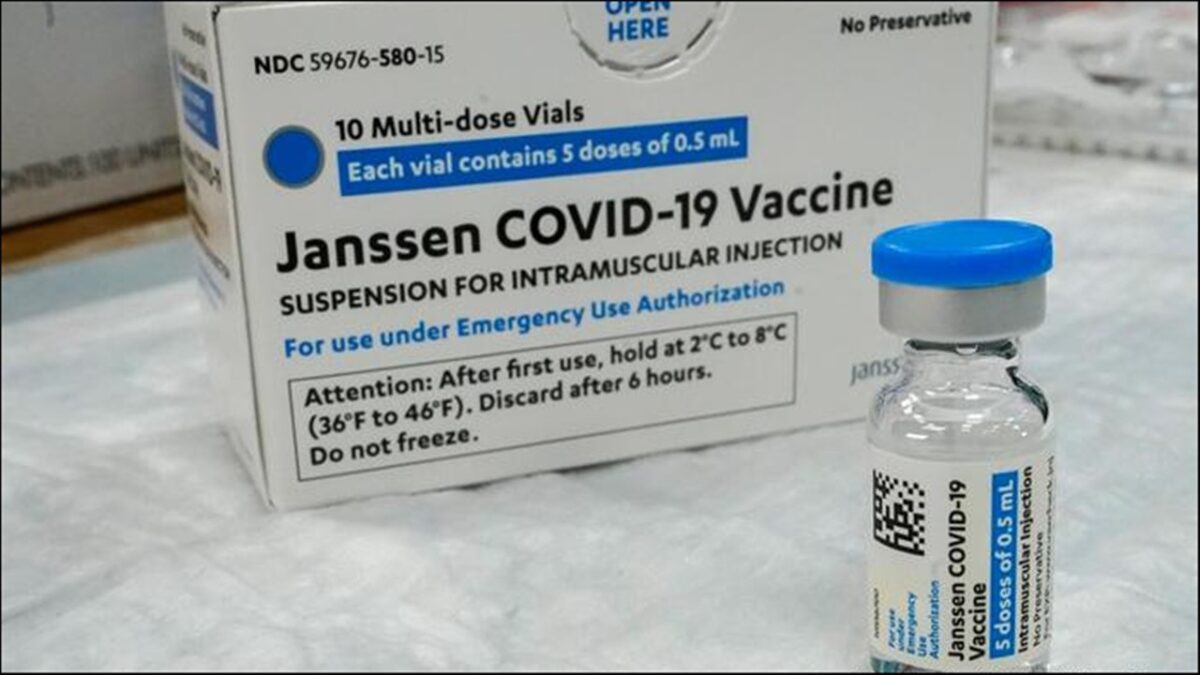
The government of Bangladesh has authorised Johnson and Johnson single dose COVID vaccine for emergency use in the country.
Johnson and Johnson had received the approval of the World Health Organisation (WHO) in March this year.
A press release of the Directorate General of Drug Administration (DGDA) on Tuesday said that the authorisation has been given after examination by the committee.
Johnson and Johnson is the sixth COVID 19 vaccine which has been approved for emergency use in Bangladesh.
Earlier, Emergency Use Authorization (EUA) has been provided to CoronaVac produced by the Chinese Company Sinovac, Pfizer mRNA, Covishield produced by the Serum Institute of India, Russian produced Sputnik V vaccine and Sinopharm produced by the Beijing Institute of Biological products.
Meanwhile, the Corona situation in Bangladesh continued to be grim with 50 deaths and 3319 fresh cases reported on Tuesday.
The sample positivity rate registered a slight fall at 14.27 percent from 14.80 percent recorded on Monday.
The death toll in Bangladesh now stands at 13,222 while the total number of infections since March last year is over 8.33 lakh.


























 WhatsApp us
WhatsApp us